IOWA STATE UNIVERSITY
Lloyd Veterinary Medical Center
Clinical Trial Service
New Clinical Trial for Canine IBD
Project Overview
This is an industry-sponsored clinical trial investigating the efficacy of a commercial product (Intesto-Guard™) in dogs with IBD. Intesto-Guard™ is an orally administered synbiotic-immunoglobulin product that has been shown to be safe when administered with no adverse effects. Anecdotal observations suggest it is efficacious in dogs with acute and chronic diarrhea.
Trial Design
- This is a double-blinded, randomized trial of 6 weeks duration in dogs with non-PLE IBD.
- Dogs with IBD will be randomized to receive one of 2 treatments:
Hydrolyzed diet + Intesto-Guard;
Hydrolyzed diet + Placebo.
- Clinical remission defined as 50% or > reduction in CIBDAI/CCECAI baseline score.
- Dogs failing to achieve remission at 2 weeks will be placed on the next sequential step-up therapy, such as cyclosporine at 5 mg/kg PO 9 24h, for remaining 4 weeks.
- Endoscopy before and 6 weeks after initiating treatment with routine (H&E) histology included in costs.
- Additional mucosal biopsies will also be collected for intestinal cell cultures.
- Trial covers all fees associated with office visits, lab work, histology and both Gl endoscopies.
- Elimination diet is provided for free for trial duration.
Client Costs
Really none for successful completion of trial. Each study participant will receive a study participation credit, not to exceed $4000 off the total bill after completion of the third visit. Out of pocket expenses of $100 (total costs) will be the only costs incurred by client.

IOWA STATE UNIVERSITY
Lloyd Veterinary Medical Center
1Department of Veterinary Clinical Sciences, 2Department of Biomedical Sciences and 3Veterinary Diagnostic Laboratory, College of Veterinary Medicine, Iowa State University, Ames, IA
Sichao Mao1, Todd Atherly1, Dana Borcherding2, Yoko Ambrosini2, Jennifer Groeltz3, Yeon-Jung Seo2, Jonathan Mochel2, Karin Allenspach1, and Albert Jergens1
Phenotypic and functional characterization of ileal organoids from dogs with inflammatory bowel disease
BACKGROUND
Canine inflammatory bowel disease (IBD) refers to a group of chronic gastrointestinal (GI) disorders of unknown cause and ill-defined pathogenesis.1 With the aim of modeling spontaneous GI disease in dogs and humans, intestinal stem cell (ISC)-derived organoids are emerging as a promising ex vivo tool in the study of IBD pathogenesis due to their three-dimensional (3D) epithelial architecture, remarkable self-renewing ability and faithful reflection of epithelial function of in vivo tissues.2 Previous studies from our lab and others have validated the cultivation of canine intestinal organoids (enteroids and colonoids) from crypt base columnar (CSC) cells of healthy dogs and characterized healthy canine organoids.3 However, little is known about the phenotypic and functional changes of canine intestinal organoids derived from dogs with IBD.
HYPOTHESIS AND STUDY AIMS
Our hypothesis was ileal organoids derived from inflamed intestinal mucosa would manifest phenotypic or functional differences from those derived from healthy dogs, while enteroids would share similarities with original tissues in phenotypic features.
The first aim was to compare the similarities and differences between healthy and diseased IBD enteroids with respect to phenotypic and functional characterization. Our second aim was to validate the utility of organoids as ex vivo tools to study the IBD pathogenesis and pharmacotherapies.
MATERIALS AND METHODS
STUDY DESIGN
We performed a retrospective study using archived paraffin-embedded ileal biopsies from two dogs diagnosed with IBD and two healthy dogs. Ileal organoids of the same dogs were revived from a bio-bank of cryopreserved enteroids or cultivated from ileal endoscopic biopsies. A panel of six phenotypic markers identified different epithelial cell lineages (LGR5+: intestinal stem cell, ALP: enterocyte, PAS: goblet cell, NeuroG3: enteroendocrine cell), epithelial barrier integrity (ZO-1) and cell proliferation (Ki-67). Functional features of IBD organoids were investigated by cystic fibrosis transmembrane conductance regulator (CFTR) organoid swelling assay to measure Cl– channel-water conductance.
RNA in situ hybridization (RNA-ISH)
We performed a retrospective study using archived paraffin-embedded ileal biopsies from two dogs diagnosed with IBD and two healthy dogs. Ileal organoids of the same dogs were revived from a bio-bank of cryopreserved enteroids or cultivated from ileal endoscopic biopsies. A panel of six phenotypic markers identified different epithelial cell lineages (LGR5+: intestinal stem cell, ALP: enterocyte, PAS: goblet cell, NeuroG3: enteroendocrine cell), epithelial barrier integrity (ZO-1) and cell proliferation (Ki-67). Functional features of IBD organoids were investigated by cystic fibrosis transmembrane conductance regulator (CFTR) organoid swelling assay to measure Cl– channel-water conductance.
Immunohistochemistry (IHC)
Paraffin-embedded sections were first deparaffinized and rehydrated, followed by antigen retrieval and blocking steps. After blocking, the sections were incubated with primary antibodies and conjugated secondary antibodies. Markers used for IHC were Ki-67, ZO-1 and PAS.
Imaging and Semi-quantitative assessment
RNAscope and IHC stained sections were visualized under ×40 magnification using an Olympus BX40 microscope (Olympus Optical Co., LTD, Japan) and imaged with an Olympus DP27 camera. A minimum of seven images were randomly obtained from different representative fields per slide. Semi-quantitative assessment of RNAscope and IHC staining was achieved by ImageJ software.
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) function assay
Enteroids were passaged and seeded in Matrigel into 24-well plates. Two days later, they were incubated in CMGF+ medium with 10 µM forskolin (CFTR potentiator) or DMSO (vehicle control). Six wells and two fields per well were adopted for imaging. Representative images were taken with Leica Application Suite (LAS) software at 0, 1, 4 h at ×5 magnification using an inverted microscope. The average enteroid area in each field was measured with ImageJ software.

Fig 1. Canine ileal organoids from healthy dogs and dogs with IBD. Representative images of differentiated 5-7-day-old ileal enteroid were obtained with Leica Application Suite (LAS) software at × 40 magnification. Scale bar: 50 µm

Table 1. Means of biomarkers expressed in healthy or IBD enteroids and tissues, including LGR5+ (intestinal stem cell), ALP (enterocyte), NeuroG3 (enteroendocrine cell), ZO-1 (epithelial barrier integrity), Ki-67 (cell proliferation) and PAS (goblet cell).
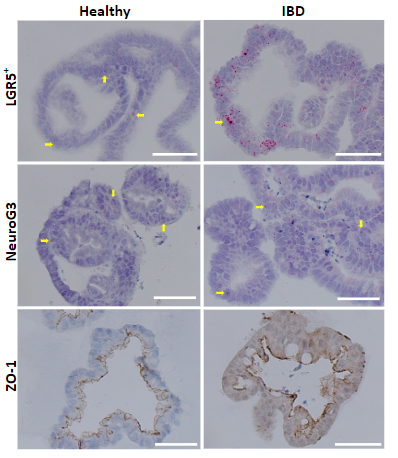

Fig 2. Representative images of RNA-ISH and IHC at ×40 magnification. Scale bar: 50 µm. NeuroG3 and PAS expression in organoids shows significant difference between healthy and IBD organoids. Expression of ZO-1 exhibits similar trend between IBD organoids and tissues.


Fig 1. Canine ileal organoids from healthy dogs and dogs with IBD. Representative images of differentiated 5-7-day-old ileal enteroid were obtained with Leica Application Suite (LAS) software at × 40 magnification. Scale bar: 50 µm
DATA ANALYSIS
Mean and standard deviation were calculated from multiple independent measurements. Two sample t-tests and multiple pairwise comparisons were performed to determine group differences.
RESULTS
- Whole tissues exhibited inflammation-mediated changes in ALP, LGR5+, NeuroG3, Ki-67, ZO-1 as anticipated.
- Significant differences in expression of phenotypic markers NeuroG3 and PAS were observed between healthy and IBD organoids (p<0.05).
- Similar trends in expression of LGR5+, NeuroG3 and ZO-1 were observed between inflamed whole tissues versus IBD organoids.
- Swelling assay (CFTR) showed that IBD organoids have functional CFTR-Cl– channels but behave differently from healthy organoids.
CONCLUSIONS
- This is the first report of phenotypic changes in intestinal organoids from dogs with IBD.
- IBD organoids show different phenotypic features as compared to healthy organoids.
- IBD organoids exhibit similar trends to tissues for LGR5+, NeuroG3 and ZO-1 cell expression. This suggests their utility in simulating tissues in an ex vivo environment for investigating IBD pathomechanisms and modeling pharmacotherapy.
- CFTR swelling assay suggests increased CFTR-Cl– channel function in IBD organoids as compared to healthy organoids.
REFERENCES
- Jergens, A. E. et al. A Scoring Index for Disease Activity in Canine Inflammatory Bowel Disease. J. Vet. Intern. Med. (2003).
- Mochel, J. P. et al. Intestinal Stem Cells to Advance Drug Development, Precision, and Regenerative Medicine: A Paradigm Shift in Translational Research HHS Public Access Author manuscript. AAPS J. (2018) 20(1): 17.
- Chandra, L. et al. Derivation of adult canine intestinal organoids for translational research in gastroenterology. BMC Biology (2019) 17:33.
Acknowledgement: This project was supported by IG Biosciences Corp.
Clinical Trial Contacts
- Dr. Karin Allenspach, allek@iastate.edu, 515-294-4900
- Dr. Al Jergens, ajergens@iastate.edu, 515-291-5192 (Primary) or 515-460-1205
- Dr. Agnes Bourgois-Mochel, Clinical Trials Coordinator, abmochel@iastate.edu, 515-294-4900
- vetmed.iastate.edu/vmc/clinical-trials